Epicyon haydeni

Quick Facts
Common Name: none
About the size of a grizzly bear, this species is the largest canid ever known.
Its known range extended across the United States from Florida to California and Oregon.
Age Range
- Middle to late Miocene epoch; Clarendonian to early Hemphilian North American land mammal ages
- About 12 to 6 million years ago
Scientific Name and Classification
Epicyon haydeni Leidy, 1858
Source of Species Name: The species name honors Dr. Ferdinand Vandeveer Hayden (1828?-1887), a geologist who explored and surveyed much of the American West. Hayden collected the type specimen of the species and many others named by Leidy while a member of the 1856-1857 Warren surveying expedition.
Classification: Mammalia, Eutheria, Carnivora, Caniformia, Cynoidea, Canidae, Borophaginae, Borophagina
Alternate Scientific Names: Canis haydeni; Aelurodon haydeni; Aelurodon haydeni validus; Tephrocyon mortifer; Aelurodon aphobus; Tomarctus mortifer; Osteoborus validus; Osteoborus ricardoensis; Aelurodon mortifer; Epicyon validus

Overall Geographic Range
Epicyon haydeni had a wide range in North America, and fossils belonging to this species have also been found in Texas, Nebraska, Montana, New Mexico, Oklahoma, California, Arizona, Kansas, Colorado, Oklahoma, Idaho, and Oregon. The type locality is in the “Valley of the Niobara River” (Leidy, 1858), probably in the Merritt Dam Member of the Ash Hollow Formation in the late Clarendonian of Nebraska (Wang et al., 1999).
Florida Fossil Occurrences
Florida fossil sites with Epicyon haydeni:
- Alachua County—Haile 19A; Love Bone Bed; McGehee Farm
- Hardee County—Fort Green Mine (#8 Dragline)
- Levy County—Mixon’s Bone Bed; Waccasassa River 6
- Polk County—Four Corners Fauna (Fort Green Mine; Nichols Mine; Nichols Mine Stream Matrix; Payne Creek Mine); Phosphoria Mine; Silver City Mine
Discussion
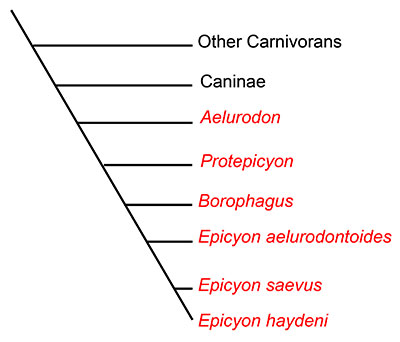
Dogs and their relatives are classified in the mammalian family Canidae. Canidae is the oldest surviving lineage in the order Carnivora, first evolving more than 40 million years ago in the late Eocene of North America. All extant canids are classified in the subfamily Caninae, but another subfamily, Borophaginae, existed in the past. Unlike dogs classified in Caninae, Borophagine dogs are commonly known as “bone-crushers” and are often compared to hyaenas (which are not in the dog family) in their food-processing behavior (Wang et al., 1999; Wang et al., 2008). While not all borophagines dogs were bone-crushers based on their anatomy, many species possessed convergently evolved dental and cranial characteristics similar to those of hyaenas. Known only from North America, borophagine dogs first appeared about 32 million years ago in the Oligocene epoch, and were dominant components of the mammalian fauna until their extinction approximately 2 million years ago in the early Pleistocene. Borophagine dogs exhibited a high level of diversity during that time, taking a variety of ecological roles. Some borophagines were hypercarnivorous with large body size, while others were smaller with omnivorous diets (Wang et al, 2004; Wang et al, 2008).Epicyon haydeni is a late-occuring borophagine dog that exemplifies one extreme end of the spectrum of borophagine diversity. The largest individuals of Epicyon haydeni were about the size of a grizzly bear, which makes this animal the largest canid ever known (Wang et al., 1999). Epicyon haydeni is very closely related to a smaller, contemporaneous species, Epicyon saevus. The genusEpicyon and the genus Borophagus, another late-occurring, large, bone-crushing borophagine (from which the subfamily name is derived), are thought to comprise a monophyleticclade probably descended from an earlier genus, Protoepicyon (Figure 2; Wang et al., 1999).
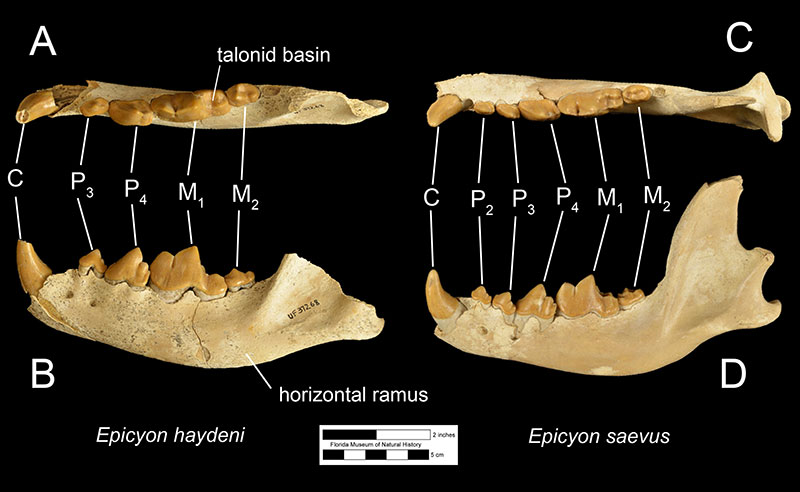
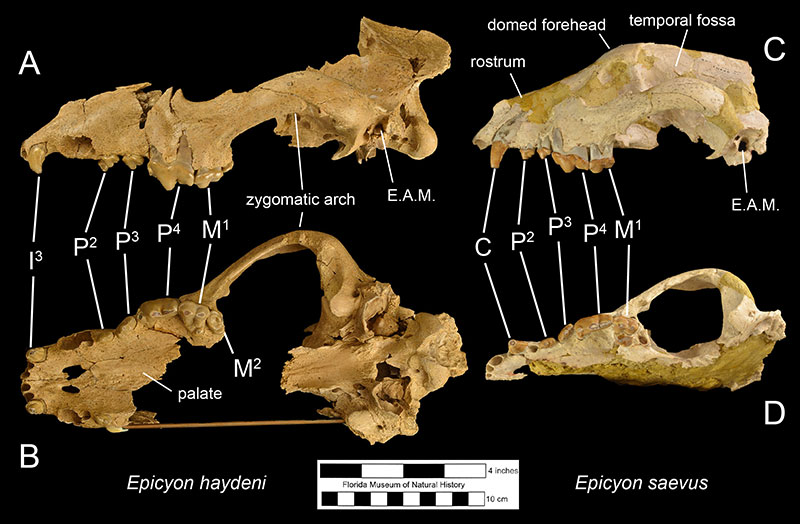
Fossils of Epicyon haydeni may be distinguished from fossils of other canids in several ways. Dentally, like many other borophagine dogs and unlike extant dogs, the lower first molar of Epicyon haydeni has a talonid basin (Figure 3; Wang et al., 2008). The teeth also reveal specializations for a hypercarnivorous, bone-crushing diet. Like another inferred bone-crusher, Borophagus, the carnassial teeth (upper fourth premolar and lower first molar) were large, as is the fourth lower premolar. Premolars anterior to the fourth in both the maxilla and mandible are small in comparison, but the molars are large relative to carnassial size (Munthe, 1989). It is thought that these relatively large cheek teeth were used to break open bones, much like hyenas do today (Wang et al, 1999; Wang et al, 2004). Many teeth of Epicyon haydeni show signs of very heavy wear, which has also been interpreted to be evidence of dental erosion caused by bone-crushing (Munthe, 1989). Borophagus and Epicyon are also similar to hyaenas in having a widened palate, shorter rostrum, and a dome-shaped forehead, but the doming of the forehead is more pronounced and the rostrum is shorter in Borophagus relative to the condition found in Epicyon (Figure 4).
The most obvious difference between Epicyon haydeni and other species of Epicyon is its larger size (Figures 3-4). Compared to Epicyon haydeni, the ramus of the mandibles of Epicyon saevus and Epicyon aelurodontoides was more gracile and shallower horizontally. In addition, the cranium of Epicyon haydeni had a relatively elongated external auditory meatus (the foramen in the skull leading to the inner ear). Several cusp characteristics on the upper and lower fourth premolar and second lower molar have been shown to be diagnostically different, as have dental proportions (Wang et al., 1999). Generally, the face of E. saevus is longer than E. haydeni, but the zygomatics are less shallow and the temporal fossa is larger in the cranium of E. haydeni.
Postcranially, Epicyon is similar to other canids in having a small clavicle, flexible back, and a digitigrade posture, which are thought to be adaptations to lenghten the distance traveled with each step. Often, an elongated stride is associated with cursorial behavior, but it appears from studies of the limb proportions and robustness of the skeleton that Epicyon was less cursorial than hyaenas or modern wolves but more cursorial than some borophagine taxa such as Aelurodon (Munthe, 1989; Munthe, 1998). This seems to suggest that Epicyon lived in more open areas than Aelurodon. It is also likely that Epicyon haydeni was less cursorial and incapable of running as long of a distance as Epicyon saevus due to its larger size and heavier, less gracile skeleton (Munthe, 1989).
Because they are found in multiple localities across North America dated to a wide range of times, fossils of Epicyon haydeni were originally assigned to many different species (Wang et al., 1999). The task of identifying Epicyon haydeni fossils is confounded by the co-occurrence with Epicyon saevus at many sites during the two species’ existence. The two species are extremely similar in anatomy except for a 16-23% size difference at any given locality where they co-occur. Further confusing the situation is the fact that both Epicyon saevus and Epicyon haydeni trend towards larger size through time, such that while body sizes at a given locality or stratum do not typically overlap, the body sizes of the geologically oldest Epicyon haydeni overlap in body size with those of the geologically youngest Epicyon saevus. That is, the smallest Epicyon haydeni from an earlier time are about the same size as the largest Epicyon saevus from a later time. This parallel trend in body size evolution between the two species is also accompanied by a parallel trend in the evolution of more pronounced doming of the frontal portion of the skull and enlargement of the fourth premolar relative to the other premolars in both species (Wang et al., 1999).
The possibility that Epicyon haydeni and Epicyon saevus represent a single, grossly sexually dimorphic species has been considered, but has been evaluated to be an unlikely scenario for several reasons. Firstly, known members of Canidae do not usually exhibit such pronounced sexual dimorphism; typical intraspecific variation in body size is around 5% for living canid species. Secondly, while the variation in dental anatomy does overlap between the two species, a higher proportion of fossils attributed to Epicyon haydeni show certain characteristic cusp morphologies and dental proportions. Thus, it seems more likely that Epicyon haydeni and Epicyon saevus were two different, but closely related, species that co-evolved toward larger body sizes but maintained a constant relative size difference with each other to fill slightly different ecological roles while co-existing in the same areas (Wang et al., 1999).
Epicyon haydeni is not known after the early Hemphilian. Wang et al. (1999) listed some specimens from the Central Florida Phosphate Distict (Bone Valley) and gave their age as late Hemphillian (Palmetto Fauna). If true, that would make those specimens the youngest known record of the species. However, there are no confirmed records of in situ recovery of its fossils with those of Palmetto Fauna species (Webb et al., 2008). Instead, these specimens were found with other species of the early Hemphillian Four Corners Fauna, so their age is similar to those from Haile 19A and McGehee Farm.
Sources
- Original Author(s): Arianna Harrington
- Original Completion Date: April 20, 2015
- Editor(s) Name(s): Richard C. Hulbert, Jr., Natali Valdes
- Last Updated On: April 23, 2015
This material is based upon work supported by the National Science Foundation under Grant Number CSBR 1203222, Jonathan Bloch, Principal Investigator. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Copyright © Florida Museum of Natural History, University of Florida