A Florida Museum of Natural History study provides new insights into the complex, shared history between blood-sucking lice and the vitamin-producing bacterial sidekicks that enable them to parasitize mammals, including primates and humans.
Lice depend on bacteria to supply essential vitamins missing from blood, their only food source. These bacterial partners live in specialized cells inside their insect hosts and pass from a female louse to her offspring. Lice could not survive without their symbiotic bacteria, and the bacteria, in turn, cannot live outside their insect hosts.

Photo by James Gathany, CDC Public Health Image Library
When their partnership began, however, and how it has evolved over time has been unclear. Previous studies suggested lice acquired and replaced their bacterial symbionts multiple times over their evolutionary history.
But a study by Florida Museum researchers Bret Boyd and David Reed found lice that parasitize primates and humans have hosted their endosymbionts continuously for at least 20 to 25 million years, aligning with the time period during which great apes and Old World monkeys shared a common ancestor.
As primates evolved, so did lice, and the evolution of their bacterial partners stayed closely in step.
The data provide a new perspective on the evolutionary tree of these symbiotic bacteria, said Boyd, who conducted the research as a doctoral student at the museum.
“While lice are highly maligned, they provide a wealth of scientific information,” said Boyd, now a postdoctoral researcher at the University of Georgia and the study’s first author. “Because these symbiotic bacteria are tied to a known evolutionary history between lice and primates, this is an ideal system for studying bacterial genome evolution.”
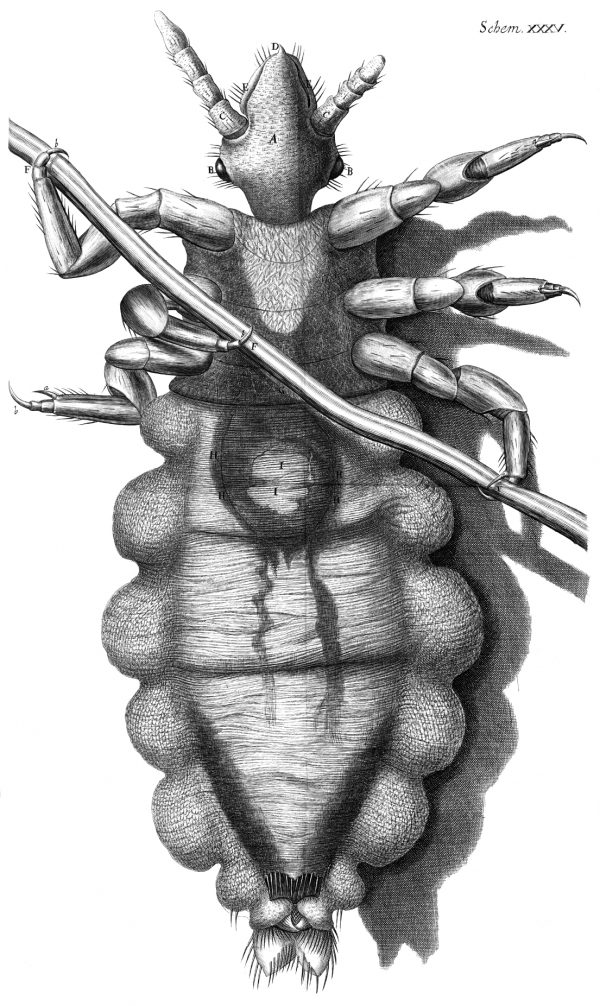
Illustration from Hooke's 1667 Micrographia
Many species of blood-sucking lice only parasitize one species of host, a specificity that can offer glimpses into primate and human evolution, said Reed, curator of mammals and associate director of research and collections at the Florida Museum.
“Certain parts of our history are murky and hard to reconstruct,” he said. “The evolution of lice and their symbiotic bacteria helps shed light on human and primate evolutionary history, providing new clues to our past.”
To gain a more complete picture of how lice and their bacterial symbionts have coevolved, the researchers sequenced and assembled genomes of endosymbionts from human, chimpanzee, gorilla and red colobus monkey lice.
They found that the bacteria’s genomes are tiny, hovering between 530,000 and 570,000 base pairs — E. coli’s genome, by comparison, is about 4.6 million base pairs.
Small genomes are a typical feature of insect symbionts, which lose much of their genome over the course of their relationships with their hosts.
Comparing different symbiont genomes, the researchers discovered evidence of extensive genome remodeling during the last 25 million years that has resulted in genes critical to louse-symbiont symbiosis being close to one another in the bacterial genome. This arrangement likely proved advantageous, as it persists in many louse symbionts today, Boyd said.
The study also showed that much of the symbiont genome is devoted to vitamin synthesis. In lice that parasitize humans, gorillas, chimpanzees and monkeys, symbionts make B vitamins crucial for basic cellular processes

Photo by James Gathany, CDC Public Health Image Library
How symbiont genomes shrank over time and which genes remain are key research questions in basic and applied sciences, Boyd said.
“The process by which symbiont genomes change is important to understanding how insects and bacteria form mutualistic relationships that can persist for tens to hundreds of millions of years,” he said. “The genes that are retained in the tiny genome provide insights into which genes are essential to maintain bacterial life.”
The study was published in Molecular Biology and Evolution and is available at http://dx.doi.org/10.1093/molbev/msx117. Funding from the National Science Foundation supported the research.
Learn more about the Mammals Collection and the Reed Lab at the Florida Museum.