Abstract
This report is the first to identify the presence of the extinct ground sloth Glossotherium tropicorum from Pleistocene-aged tar pit deposits in Trinidad. G. tropicorum is known primarily from extensive skeletal material recovered from the Pleistocene of western Ecuador and Peru. Our identification is based on an isolated left petrosal bone, which exhibits a variety of derived diagnostic features of mylodontid sloths in general, e.g., the presence of an epitympanic recess, and the genus Glossotherium in particular, including a reduced anteroventral process of the tegmen tympani. This represents only the second published record of the genus Glossotherium from outside continental South America, and is a substantial range extension for the species G. tropicorum. The present study also represents the first published description of a mylodontid sloth fossil from Trinidad. The specimen is part of a collection that was initially documented in an unpublished Master’s thesis, and that includes additional sloths and other Pleistocene mammals. This fauna confirms the presence of extensive savanna-type habitats on Trinidad during the Pleistocene, and is consistent with the presence of a land bridge connection between the island and mainland South America at that time.
Key Words: Sloths, Glossotherium, Trinidad, Pleistocene, tar pit, ear region, skull, anatomy
Download Vol. 58, No.3, high resolution
Introduction
Sloths are herbivorous members of the Xenarthra. Living sloths comprise a small clade of two genera and six species placed in two different families confined to the rainforests of South and Central America and a Caribbean island, (Anderson and Handley, 2002; Gaudin, 2004; Varela et al., 2018; Preslee et al., 2019; Delsuc et al., 2019). They represent only a small remnant of a much more diverse radiation that was found throughout the Americas, from Alaska to Patagonia, including some of the islands of the Caribbean, and numbering at least 100 genera (e.g., McKenna and Bell, 1997; McDonald and De Iuliis, 2008; Pujos et al., 2012). The majority of these extinct sloths were terrestrial, and had a body size much greater than that of living sloths, some at least as large as modern-day elephants, and likely displayed more dietary diversity than living forms (e.g., McDonald and De Iuliis, 2008; Bargo and Vizcaíno, 2009; Pujos et al., 2012; Resar et al., 2013; Dantas et al., 2017). However, all large terrestrial sloths became extinct in the megafaunal extinction event at the end of the Pleistocene (McDonald and De Iuliis, 2008), except for the Antillean taxa (Steadman et al., 2005). Our knowledge of this great sloth radiation has increased rapidly over the past few decades, and numerous new taxa have been recognized (e.g., McDonald and De Iuliis, 2008; Pujos et al., 2012; Varela et al., 2018; Boscaini et al., 2019). One such recently recognized taxon is a mylodontine sloth Glossotherium tropicorum, which is known from localities in northwestern South America, including parts of southwestern Ecuador and the northernmost portions of Peru (Fig. 1; De Iuliis et al., 2017).
The subject of the present report is a single fossil specimen housed in the collections of the Florida Museum of Natural History, recovered from tar pit deposits on the present-day island of Trinidad, which is situated just off the Venezuelan coast of northern South America (Fig. 1). The fossil is an isolated petrosal bone from a mylodontine sloth, and it is our contention that this specimen likely pertains to Glossotherium tropicorum.
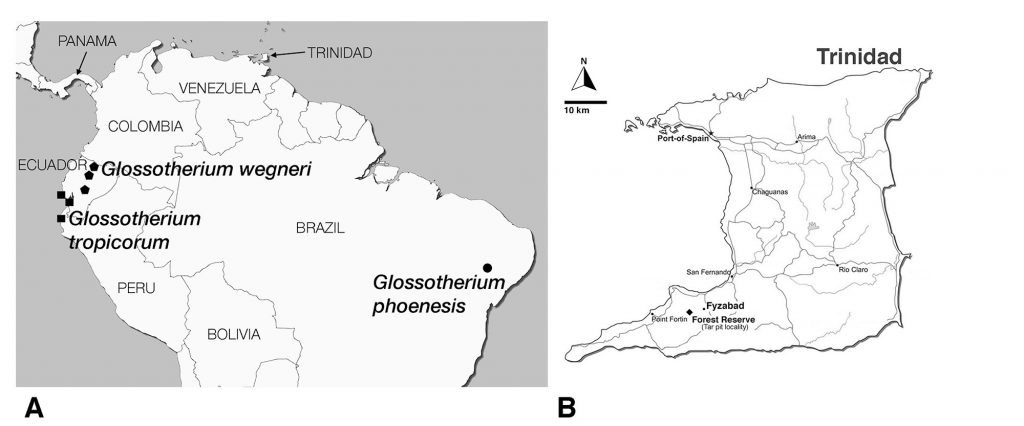
Although Trinidad is an island today, it lies on the coastal shelf of South America as the closest member of the Antillean chain to mainland South America. Given the repeated glaciation events during the Pleistocene epoch (2.6 Ma to 11.7Ka), and the concomitant sea level changes (Kurtén and Anderson, 1980; Pielou, 1981; Ogg et al., 2016), it is likely that Trinidad was connected repeatedly to South America over the course of its recent geological history (Comeau, 1991; Mychajliw et al., 2020). Moreover, Pleistocene climate oscillations changed not only the physiographic features of the landscape, but also the structure of ecological communities. Areas that today are largely forested were at various points in the past covered by savannah type environments (Comeau, 1991; Webb, 1991), and this likely includes Trinidad (Wing, 1962; Comeau, 1991; Mychajliw et al., 2020). Given this, perhaps it would not be surprising to find a large, terrestrial, grazing sloth in the fossil record of the island, and yet, such reports are scarce.
The likelihood of finding such records is enhanced by the presence of tar pits on the island (=asphaltic deposits or asphaltic seeps, Wing, 1962; Mychajliw et al., 2020 – Wing refers to these as “oil sand” deposits). Tar pits represent a particularly valuable source of information on the paleoecology of the Pleistocene (Harris, 2015). These sites often contain a large abundance and diversity of very well-reserved fossils. Tar pits are found in all parts of the world, and may closely reflect the ecosystem of their surrounding region. The sticky tar can act as a trap to ensnare passing animals (McDonald et al., 2015; Mychajliw et al., 2020) and other parts of the biota, a function that may be facilitated by water or plant debris accumulating on the surface of the tar. Fossils of Glossotherium tropicorum have been recovered from several tar pit deposits in Ecuador and Peru (Fig. 1; De Iuliis et al., 2017).
The goal of the present study is to report on the identification of a fossil of Glossotherium tropicorum, a typically South American large bodied, terrestrial, grazing extinct sloth, typical of South America, in a Pleistocene-aged tar pit deposit from Trinidad. This will enhance our understanding of the biogeography of this sloth species, as well as the Quaternary biogeographic history of Trinidad and nearby portions of northern South America.
Materials and Methods
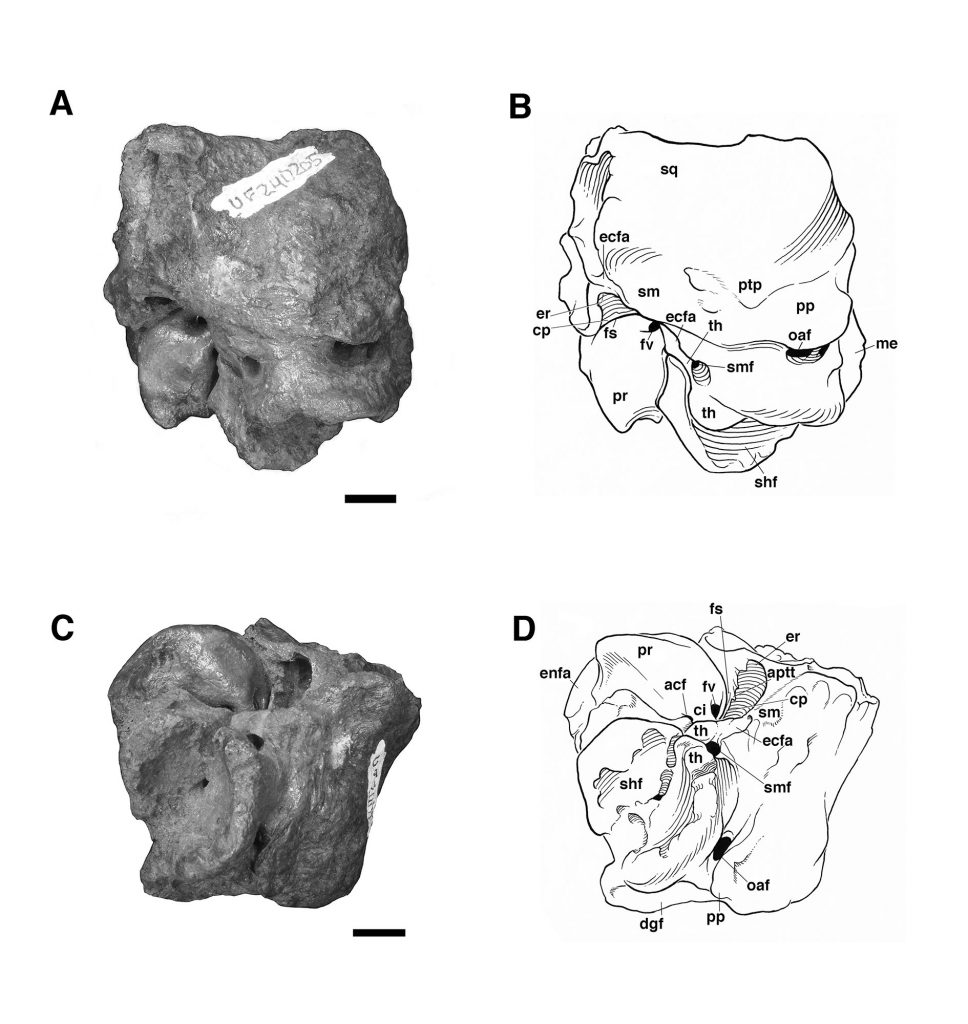
The fossil mylodontine sloth specimen is an isolated left petrosal (UF 240205, Figs. 2–3) housed at the Florida Museum of Natural History (University of Florida, Gainesville, FL). It was part of a collection recovered and described by Wing (1962), though the specimen itself was not reported in Wing’s (1962) unpublished Master’s thesis. Wing’s study area was in the Texaco Forest Reserve near Fyzabad in southwestern Trinidad and dates to the late Pleistocene (Fig. 1; 34Ka, Wing, 1962; Lujanian SALMA, Woodburne et al., 2014). This locality has yielded other Pleistocene mammal remains (Wing, 1962), including gomphotheriid proboscidean teeth, a nearly complete skeleton of the armored cingulate Glyptodon sp., a partial tooth and other skeletal remains assigned to the giant megatheriine sloth “Megatherium americanum” (almost certainly pertaining instead to the species Eremotherium laurillardi; see Cartelle and De Iuliis, 1995), and a single tooth ascribed to the genus “Mylodon.” The latter likely pertains to Glossotherium tropicorum as well. The Forest Reserve fauna has not been the subject of a published study, the only report being Wing’s (1962) thesis. The fossils themselves are housed at the Florida Museum of Natural History and the American Museum of Natural History (Wing, 1962).
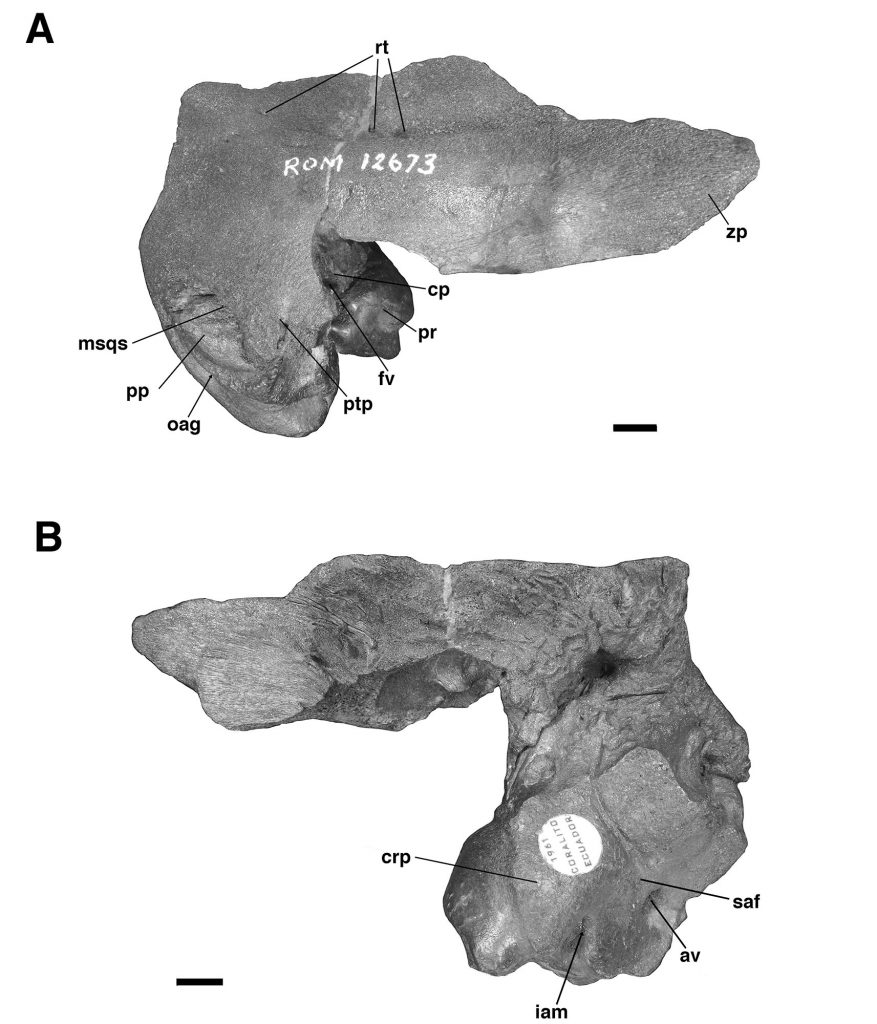
For comparative purposes, we examined petrosals of Glossotherium tropicorum recovered from tar pits in Ecuador and Peru (De Iuliis et al., 2017) and housed at the Royal Ontario Museum (Toronto, Canada), including a partial skull (ROM 3146), an isolated right petrosal and squamosal bone (ROM 12673, Figs. 4–5), and an isolated left petrosal (ROM 12674, Fig. 5), the latter two likely from juvenile individuals based on bone texture and the presence of open sutures. We also compared the Trinidad fossil to published description of other mylodontine petrosals (Guth, 1961; Patterson et al., 1992; Boscaini et al., 2018; Román-Carrión and Brambilla, 2019).
Abbreviations: ROM, Royal Ontario Museum, Toronto, Canada; SALMA, South American Land Mammal Age; UF, Florida Museum of Natural History, University of Florida, Gainesville, USA.
SYSTEMATIC PALEONTOLOGY
XENARTHRA Cope, 1889
PILOSA Flower, 1883
FOLIVORA Delsuc et al., 2001
MYLODONTIDAE Gill, 1872
GLOSSOTHERIUM Owen, 1840
GLOSSOTHERIUM TROPICORUM De Iuliis et al., 2017
Referred Material—Isolated right petrosal bone (UF 240205) (Figs. 2—3).
Locality—Texaco Forest Reserve near Fyzabad, southwest Trinidad (Fig. 1).
Age—34 Ka (Wing, 1962; but see Mychajliw et al., 2020), Lujanian SALMA
Description
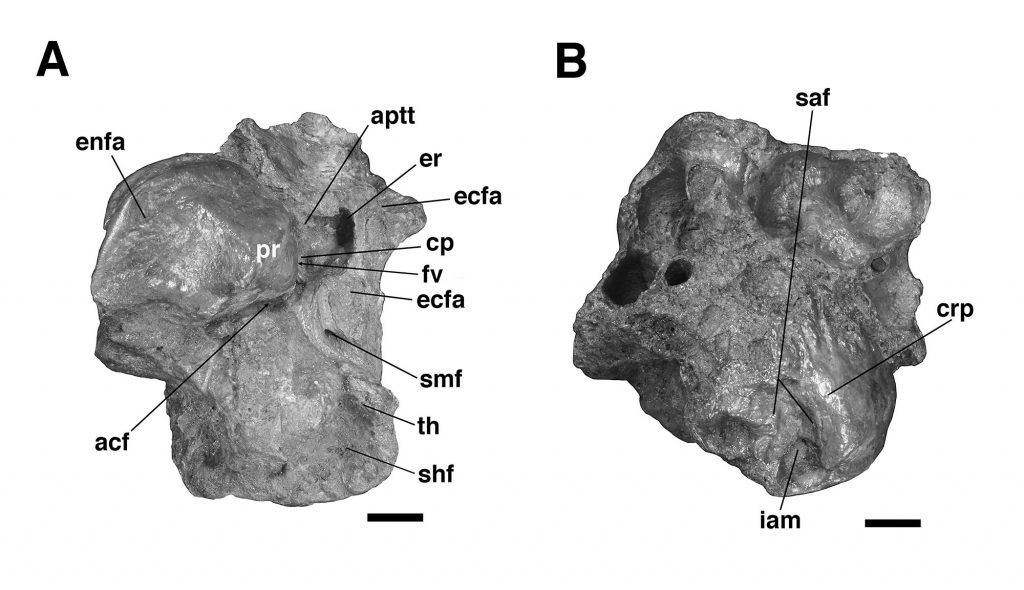
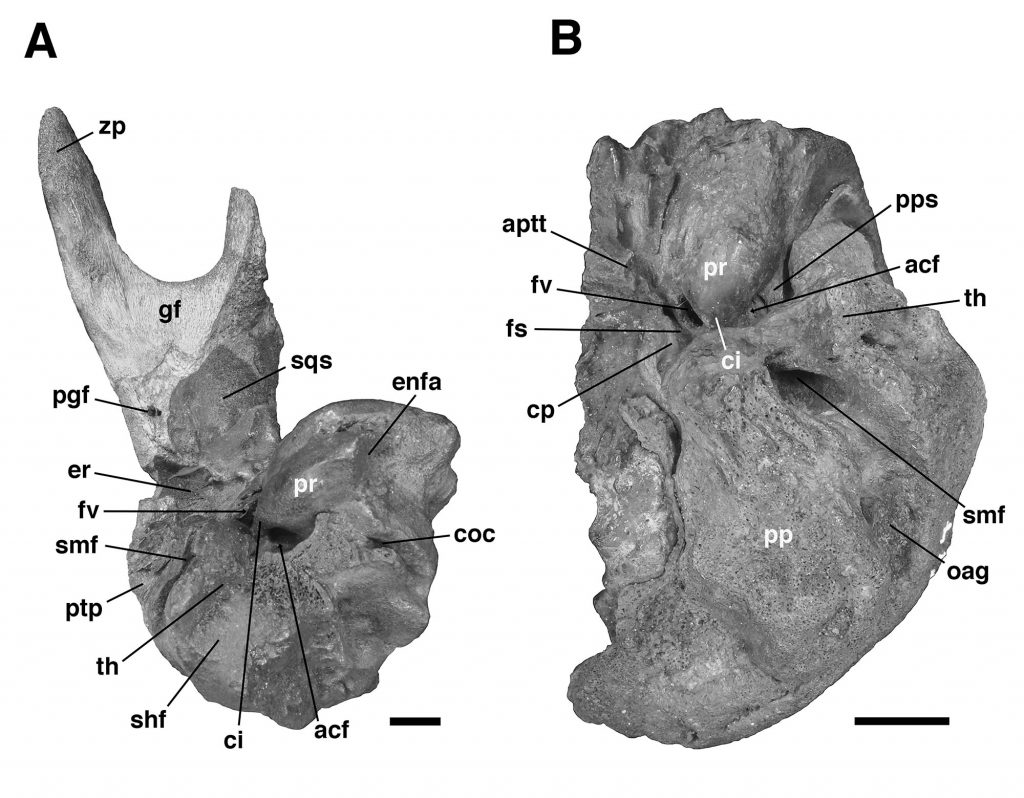
The specimen UF 240205 includes most of the pars cochlearis of the left petrosal (housing the cochlear duct and saccule) and tympanohyal, but is missing most of the posterodorsal part of the pars canalicularis (for the utricle and semicircular canals), including the large occipital exposure of the mastoid generally present in mylodontid sloths (Figs. 2–3; Patterson et al., 1992; Boscaini et al., 2018). A small portion of the squamosal is also present, including the posttympanic process that unites with the paroccipital process of the petrosal to form the “mastoid process” of sloths (Patterson et al., 1992; Boscaini et al., 2018). It is nearly identical to the right petrosal and squamosal of Glossotherium tropicorum (ROM 12673, Figs. 4–5) in most respects, except for some age-related differences – the latter specimen pertaining to a juvenile (based on the texture of the bone and its open sutures, e.g., between the posttympanic process of the squamosal and the paroccipital process of the petrosal), whereas UF 240205 appears fully adult, though nearly identical in size to ROM 12673.
In all specimens of G. tropicorum examined, including UF 240205, the pars cochlearis is dominated by an anteroposteriorly narrow, dorsoventrally elongated promontorium which is convex laterally, similar to the condition in most sloths (Patterson et al., 1992; Gaudin, 1995, 2004). In all specimens, the promontorium extends into a rounded ventral point, medial to which the ventral surface bears a strong concavity (Figs. 3, 5). This concavity either serves as the attachment surface of the missing entoympanic element, or the roof of the sulcus for the internal carotid artery, as described in G. robustum by Boscaini et al. (2018), or perhaps it serves both functions. Dorsally, the promontorium bears the two openings typical of mammalian petrosals, the fenestra vestibuli and the aperture for the cochlear fossula, which contains the fenestra cochleae (Wible, 2010; Boscaini et al., 2018). The former opening is ovate anteroposteriorly in UF 240205, with a nearly identical stapedial ratio (sensu Segall, 1970; =1.65) to that of ROM 12674 (=1.72), whereas the fenestra vestibuli of ROM 12673 is somewhat more elongated (stapedial ratio = 1.90). The fenestra cochleae of UF 240205 faces posteriorly and somewhat laterally, as is the case in other mylodontine sloths (Gaudin, 1995), but curiously, lacks a medial groove extending toward the entotympanic. This is another feature of the Mylodontinae, and is present in G. tarijense (Gaudin, 1995), G. robustum (Boscaini et al., 2018), and G. tropicorum ROM 12674, but is also missing in ROM 12673.
Dorsal and lateral to the fenestra vestibuli lies the deep facial sulcus for the facial nerve (CN VII), walled laterally by a strong crista parotica in all specimens examined in the present study. The crista parotica of UF 240205 in turns forms the medial wall to a large, well-preserved depression, slightly ovate anteroposteriorly, the epitympanic recess, with a smaller depression tucked into its posteromedial corner, the fossa incudis. This arrangement is typical of Mylodontidae, in contrast to other sloths in which an epitympanic sinus is present. The rostral end of the crista parotica in UF 240205 appears to bear a small anteroventral process of the tegmen tympani (Figs. 2C–D, Fig. 3A), although the size and shape of the process are somewhat obscured by matrix. Although not well preserved in the other G. tropicorum specimens, a similar process is present in G. robustum (Boscaini et al., 2018), and was identified as a synapomorphy of Lestodon, Glossotherium, and Paramylodon by Gaudin (1995).
At the opposite end of the crista parotica in UF 240205, the crest joins the base of a large tympanohyal element, which expands distally and extends ventrolaterally, its circular distal surface forming much of the stylohyal fossa so characteristic of sloths (Gaudin, 1995, 2004). Immediately lateral to the shaft of the tympanohyal is the stylomastoid foramen, situated immediately anterior to the stylohyal fossa and opening into a groove the extends ventrolaterally. The position of this aperture is a distinctive feature of mylodontine sloths, whereas the orientation of the groove is characteristic of Mylodontidae as a whole (Gaudin, 1995). The stylohyal fossa itself is oriented ventrolaterally in UF 240205, as in other mylodontids (Gaudin, 1995), and is circular in outline as preserved, as in most sloths, though it is missing the portions normally contributed by the entotympanic and exoccipital elements. In posterior view the stylohyal fossa is V-shaped, divided by a deep longitudinal groove (Fig. 2). This unusual arrangement is also present in G. tropicorum ROM 3146. This feature does not appear to be present in G. robustum (Bosciani et al., 2018), G. wegneri (Román-Carrión and Brambilla, 2019) or G. phoenesis (Cartelle et al., 2019), based on the published illustrations, and may be the best indication of the taxonomic affinities of UF 240205.
Dorsal and lateral to the stylohyal fossa in UF 240205 is a second large aperture, the ventral opening for the occipital artery (Fig. 2). This opening lies just medial to the paroccipital process of the petrosal, which is distinguishable by suture from the posttympanic process of the squamosal in ROM 12673 (Fig. 4A – note the two fuse to form the so-called “mastoid process” of Patterson et al. [1992] and Gaudin [1995]). Cartelle et al. (2019) note that this opening is positioned more ventrally in G. robustum than in G. tropicorum or G. phoenesis, because the occipital artery is more fully enclosed by its canal. The condition in UF 240205 resembles that of the latter two taxa, with the ventral-most portion of the occipital artery exposed in an open groove that connects anteriorly to the groove emerging from the stylomastoid foramen (as in other mylodontines; see Patterson et al., 1992; Gaudin, 1995). This feature in G. wegneri is difficult to assess based on published photographs, but it may vary. The specimen photographed by Román-Carrión and Brambilla (2019, figure 8A) appears to resemble G. robustum, but at least one specimen photographed by De Iuliis et al. (2020, Appendix 2B & C) seems to exhibit a more open groove, though its morphology appears anomalous relative to the other specimens.
Lastly, the medial surface of the petrosal is also available for examination in UF 240205 (Fig. 3B). It strongly resembles the morphology described and illustrated for G. robustum (Boscaini et al., 2108), as well as that observed in the two juvenile specimens of G. tropicorum (ROM 12673 and 12674; Fig. 4B). In all these specimens, there is a large aperture for the aqueductus vestibuli in the middle of the broad, shallow subarcuate fossa. This aperture is floored by a medially directed crest that also forms the floor of the more anteriorly situated internal acoustic meatus. In all these Glossotherium specimens, the deep and undivided internal acoustic meatus faces not only posteromedially, as is typical for sloths, but somewhat ventrally as well, with a horizontal ventral margin to the aperture, and an arched dorsal margin. The shape of this arch is somewhat variable: V-shaped in ROM 12674, variably quadrangular or semicircular in G. robustum (Boscaini et al., 2018), and semicircular in both UF 240205 and ROM 12673.
Discussion
It is clear from its morphology that the isolated petrosal and squamosal, UF 240205, recovered from tar pit deposits on the island of Trinidad, is a mylodontid sloth, and more specifically, a member of the subfamily Mylodontinae, based in particular on: 1) the presence and morphology of its epitympanic recess; 2) the shape and orientation of its stylohyal fossa; and, 3) the position and orientation of two of its foramina, the stylomastoid foramen and fenestra cochleae. In addition, the shape of its promontorium, the morphology of the medial surface of the petrosal, the presence of a small anteroventral process of the tegmen tympani, and its geographic location are all strongly indicative of its provenance in the genus Glossotherium. There are currently five species recognized within this genus: G. robustum, G. tarijense, G. wegneri, G. tropicorum, and G. phoenesis (Boscaini et al., 2019; De Iuliis et al. 2020). The first two are known largely from the southern parts of South America (McAfee, 2009; Pujos et al., 2016; Boscaini et al., 2018 – though there is a record ascribed to G. robustum from northern Peru in Pujos et al., 2016), and thus it seems unlikely that our petrosal pertains to one of these two species. G. wegneri is a high elevation species know only from the Andes mountains of Ecuador (De Iuliis et al. 2020). Although present in northern South America and geographically the closest species to Trinidad (Fig. 1), its restriction to high altitudes makes it equally unlikely to have been present in northern coastal areas adjoining Trinidad. G. tropicorum is from coastal regions of northern Peru and southern Ecuador, whereas G. phoenesis is endemic to easternmost Brazil. Based both on biogeography, topography and on the anatomy of the canal for the occipital artery, it seems likely that UF 240205 pertains to one of these latter two species. G. tropicorum is geographically closer than G. phoenesis, and also shares with UF 240205 an unusual morphology of the stylohyal fossa, both marked by a deep longitudinal groove. On this basis, we assign UF 240205 to the latter species.
If this taxonomic assignment is indeed correct, this new record represents an important range extension for G. tropicorum, which is currently known with certainty only from localities in northwesternmost Peru and southwestern Ecuador (De Iuliis et al., 2017), in the westernmost, coastal part of northern South America. This results in a range extension of more than 3500 km. It is worth noting that De Iuliis et al. (2017) identify unconfirmed specimens assigned to G. tropicorum from Panama (Gazin, 1957; 2100 km from Trinidad) and northern Venezuela (Bocquentin-Villanueva, 1979; 1200 km from Trinidad). Pujos et al. (2016) also note the presence of Glossotherium sp. remains from Venezuela, which, if confirmed as G. tropicorum, might serve as a further geographic link between our specimen and the mainland records of this species. Moreover, our new record, along with the other unconfirmed records, suggest that the range of G. tropicorum may have encompassed much of northern, or at least northwestern, South America.
In addition to extending the range of G. tropicorum, this specimen constitutes only the second record of the genus Glossotherium from outside continental South America (the first being Gazin’s [1957] record from Panama). As noted in Boscaini et al. (2019), the distinction between the genus Paramylodon and Glossotherium is largely a geographic one, with the former occupying North America and the latter occupying South America. It would appear, however, that the historic range of Glossotherium extended beyond modern South America, albeit not a great distance. This (and the other Trinidad Pleistocene sloth records) also represents a second, much later incursion by sloths into the islands of the Caribbean, separate from the middle Cenozoic immigration event that produced the endemic radiation of Antillean megalonychid sloths (Iturralde-Vinent and MacPhee, 1999; McDonald and De Iuliis, 2008; Phillipon et al., 2020)
This new record of G. tropicorum represents the first published, detailed description and illustration of a mylodontid sloth fossil from the island of Trinidad, and one of very few reports of sloth fossils of any kind from the island. Wing (1962), in her Master’s thesis, previously documented the presence of fossil sloths on Trinidad, but she did not illustrate any specimens, and never published her findings. She reports the presence of the genus “Mylodon” based on an isolated molar, but given the new data from the present study, it seems likely that the molar derives from Glossotherium tropicorum instead. MacPhee and Reguero (2010) published an illustration of tooth histology from a specimen of Glossotherium (AMNHVP 143455, listed as “Glossotherium sp.”, but likely G. tropicorum) from Trinidad, but included no real description of the specimen. Wing (1962) also reported the presence of the megatheriid sloth “Megatherium” based on a partial tooth and other isolated skeletal remains, but based on what we know of megatheriid biogeography, these were almost certainly specimens of Eremotherium laurillardi (Cartelle and De Iuliis, 1995, 2006; Pujos et al., 2016). Schaub (1935) had earlier recorded the presence of this extinct sloth on Trinidad, based on an isolated partial astragalus that he described but did not figure.
Lastly, the presence of G. tropicorum on Trinidad in the Pleistocene provides further confirmation of the presence of open habitats on the island at this time, as well as the existence of a savannah corridor that connected South America to surrounding islands and the Central American isthmus during the Great American Biotic Interchange (GABI; Comeau, 1991; Webb, 1991; Mychajliw et al., 2020). The change in flora from savannah to tropical evergreen and deciduous forest is documented in the fossil record of Trinidad, as reflected particularly in midden deposits recovered from the island (Wing, 1962; Comeau, 1991). The record also confirms that the plant life of Trinidad oscillated between the tropical forest typical of modern Trinidad during interglacial periods, and drier deciduous forest and savannah during colder and more arid periods corresponding to glacial intervals (Wing, 1962; Comeau, 1991; Mychajliw et al., 2020). This is reflected in the faunal remains, as indicated by the presence of other savannah-dwelling Pleistocene mammal species, including gomphotheres and glyptodonts (Blair, 1927; Wing, 1962; Sánchez-Chillón et al., 2003; Vizcaíno et al., 2011; Mychajliw et al., 2020). Unsurprisingly, the Trinidad flora and fauna resemble South American plant and animals during the Pleistocene (Blair 1927; Wing, 1962; Comeau, 1991; Mychajliw et al., 2020), likely due both to their proximity and intermittent land bridge connections between the island and the mainland.
Conclusion
The morphology of an isolated petrosal recovered from Pleistocene-aged tar pit deposits on the island of Trinidad strongly indicate a taxonomic provenance with the large bodied, terrestrial extinct sloth species Glossotherium tropicorum. This represents the first published description of a mylodontid sloth fossil from Trinidad, and represents a significant range extension for the species G. tropicorum, as well as only the second record of the well-known, widespread genus Glossotherium from outside mainland South America. It also represents a late Cenozoic emigration of sloths into the Caribbean, distinct from the much earlier event that led to the endemic radiation of Antillean megalonychid sloths. Finally, this record provides further confirmation of the presence of savannah-like, open habitats on Trinidad during the Pleistocene, as part of the savannah corridor linking North and South America during the GABI.
Acknowledgments
We thank Richard Hulbert and Jonathan Bloch (Florida Museum of Natural History, University of Florida, Gainesville, FL, USA) for the loan of the specimen that served as the subject of this report, as well as Gerry De Iuliis and Kevin Seymour (Royal Ontario Museum, Toronto, Canada) for their assistance with the loan of G. tropicorum specimens that served as critical comparative material. We also thank François Pujos and Alberto Boscaini, who served as reviewers for the manuscript, and the editor, Richard Hulbert, for their comments, which greatly improved the quality of this manuscript. This research was supported in part by the Bramblett Gift Fund and the Department of Biology, Geology & Environmental Science, University of Tennessee at Chattanooga.
Literature Cited
Anderson, R. P. and C. O. Handley. 2001. A new species of three-toed sloth (Mammalia: Xenarthra) from Panama, with a review of the genus Bradypus. Proceedings-Biological Society of Washington 114(1):1-33.
Blair, K. G. 1927. Insect remains from oil sand in Trinidad. Transactions of the Royal Entomological Society of London 75(1):137–142.
Bocquentin-Villanueva, J. 1979. Mammifères fossiles du Pléistocène supérieur de Muaco, État de Falcón, Venezuela. Unpublished Ph.D. Dissertation. Université Pierre et Marie Curie, Paris, France, 112 p.
Boscaini, A., D. A. Iurino, G. Billet, L. Hautier, R. Sardella, G. Tirao, T. J. Gaudin, and F. Pujos. 2018. Phylogenetic and functional implications of the ear region anatomy of Glossotherium robustum (Xenarthra, Mylodontidae) from the late Pleistocene of Argentina. The Science of Nature 105:28. doi:10.1007/s00114-018-1548-y
Boscaini, A., F. Pujos, and T. J. Gaudin. 2019. A reappraisal of the phylogeny of Mylodontidae (Mammalia, Xenarthra), and the divergence of mylodontine and lestodontine sloths. Zoologica Scripta 48(6):691–710. doi: 10.1111/zsc.12376
Cartelle, C., and G. De Iuliis. 1995. Eremotherium laurillardi, the Panamerican late Pleistocene megatheriid sloth. Journal of Vertebrate Paleontology 15(4):830–841. doi: 10.1080/02724634.1995.10011265
Cartelle, C., and G. De Iuliis. 2006. Eremotherium laurillardi (Lund) (Xenarthra, Megatheriidae), the Panamerican giant ground sloth: taxonomy aspects of the ontogenetic development of the skull and dentition. Journal of Systematic Paleontology 4(2):199–209. doi: 10.1017/S1477201905001781
Cartelle, C., G. De Iuliis, A. Boscaini, and F. Pujos. 2019. Anatomy, possible sexual dimorphism, and phylogenetic affinities of a new mylodontine sloth from the late Pleistocene of intertropical Brazil. Journal of Systematic Palaeontology 17(23):1957–1988. doi:10.1080/14772019.2019.1574406
Comeau, P. L. 1991. Geological events influencing natural vegetation in Trinidad. Living World Journal of the Trinidad and Tobago Field Naturalists Club 1991–1992:29–38.
Dantas, M. A. T., A. Cherkinsky, H. Bocherens, M. Drefahl, C. Bernardes, and L. de Melo França. 2017. Isotopic paleoecology of the Pleistocene megamammals from the Brazilian Intertropical Region: Feeding ecology (d13C), niche breadth and overlap. Quaternary Science Reviews 170:152–163. doi:10.1016/j.quascirev.2017.06.030
De Iuliis, G., C. Cartelle, H. G. McDonald, and F. Pujos. 2017. The mylodontine ground sloth Glossotherium tropicorum from the late Pleistocene of Ecuador and Peru. Papers in Palaeontology 3(4):613–636. doi:10.1002/spp2.1088
De Iuliis G., A. Boscaini, F. Pujos, R. K. McAfee, C. Cartelle, L. J. S. Tsuji, and L. Rook. 2020. On the status of the giant mylodontine sloth Glossotherium wegneri (Spillmann, 1931) (Xenarthra, Folivora) from the late Pleistocene of Ecuador. Comptes Rendus Palevol 19(12):215-232. doi:10.5852/cr-palevol2020v19a12
Delsuc, F., M. Kuch, G. C. Gibb, E. Karpinski, D. Hackenberger, P. Szpak, J. G. Martínez, J. I. Mead, H. G. McDonald, R. D.E. MacPhee, G. Billet, L. Hautier, and H. N. Poinar. 2019. Ancient mitogenomes reveal the evolutionary history and biogeography of sloths. Current Biology 29(12):2031–2042. doi:10.1016/j.cub.2019.05.043
Gaudin, T. J. 1995. The ear region of edentates and the phylogeny of the Tardigrada (Mammalia, Xenarthra). Journal of Vertebrate Paleontology 15(3):672–705. doi:10.1080/02724634.1995.10011255
Gaudin, T. J. 2004. Phylogenetic relationships among sloths (Mammalia, Xenarthra, Tardigrada): the craniodental evidence. Zoological Journal of the Linnean Society 140(2):255–305. doi:10.1111/j.1096-3642.2003.00100.x
Gazin, C. L. 1957. Exploration for the remains of giant ground sloths in Panama. Smithsonian Report for Panama 1956:341–354.
Guth, C. 1961. La région temporale des édentés. Unpublished Ph.D. Dissertation. L’Université de Paris, Paris, France. 192 p.
Harris, J. M. 2015. La Brea and Beyond: The Paleontology of Asphalt-Preserved Biotas. Natural History Museum of Los Angeles County Science Series 42:1–176.
Iturralde-Vinent, M. A., and R. D. E. MacPhee. 1999. Paleogeography of the Caribbean region: implications for Cenozoic biogeography. Bulletin of the American Museum of Natural Hisotry. 238:1-95.
Kurtén, B., and E. Anderson. 1980. Pleistocene Mammals of North America. Columbia University Press, New York, USA, 442 p.
MacPhee, R. D. E., and M. A. Reguero. 2010. Reinterpretation of a middle Eocene record of Tardigrada (Pilosa, Xenarthra, Mammalia) from La Meseta Formation, Seymour Island, West Antarctica. American Museum Novitates 3689:1–21. doi:10.1206/703.1
McAfee, R. K. 2009. Reassessment of the cranial characters of Glossotherium and Paramylodon (Mammalia: Xenarthra: Mylodontidae). Zoological Journal of the Linnean Society 155:885–903. doi:10.1111/j.1096-3642.2008.00468.x
McDonald, H. G., and G. De Iuliis. 2008. Fossil history of sloths. Pp. 39–55 in S. F. Vizcaíno and W. H. Loughry, eds. The Biology of the Xenarthra. University Press of Florida, Gainesville, USA.
McDonald, H. G., J. M. Harris, and E. Lindsey. 2015. Introduction. Pp. 1–4 in J. M. Harris, ed. La Brea and Beyond: The Paleontology of Asphalt-Preserved Biotas. Natural History Museum of Los Angeles County Science Series, 42, Los Angeles, USA.
McKenna, M.C., and S.K. Bell. 1997. Classification of Mammals Above the Species Level. Columbia University Press, New York, USA, 631 p.
Michajliw, A. M., R. S. Mohammed, K. A. Rice, A. B. Farrell, A. D. Rincón, R. McAfee, H. G. McDonald, and E. L. Lindsey. 2020. The biogeography of “breas”: contextualizing the taphonomy, ecology, and diversity of Trinidad’s asphaltic fossil record. Quaternary Science Reviews 232:106179. doi:10.1016/j.quascirev.2020.106179
Ogg, J. G., G. Ogg, and F. M. Gradstein. 2016. A Concise Geologic Time Scale. Elsevier Press, New York, USA, 234 p.
Patterson, B., W. Segall, W. D. Turnbull, and T. J. Gaudin. 1992. The ear region in xenarthrans (=Edentata, Mammalia). Part II. Sloths, Anteaters, Palaeanodonts, and a Miscellany. Fieldiana, Geology new series 24:1–79. doi:10.5962/bhl.title.3466
Philippon, M., J.-J. Cornée, P. Münch, D. J. J. van Hinsbergen, M. BouDagher-Fadel, L. Gailler, L. M. Boschman, F. Quillevere, L. Montheil, A. Gay, J. F. Lebrun, S. Lallemand, L. Marivaux, P.-O. Antoine, with the GARANTI Team. 2020. Eocene intra-plate shortening responsible for the rise of a faunal pathway in the northeastern Caribbean realm. PLoS one 15(10):e0241000. doi:10.1371/journal.pone.0241000
Pielou, E.C. 2008. After the Ice Age: The Return of Life to Glaciated North America. University of Chicago Press, Chicago, USA, 366 p.
Presslee, S., G. J. Slater, F. Pujos, A. M. Forasiepi, R. Fischer, K. Molloy, M. Mackie, J. V. Olsen, A. Kramarz, M. Taglioretti, F. Scaglia, M. Lezcano, J. L. Lanata, J. Southon, R. Feranec, J. Bloch, A. Hajduk, F. M. Martin, R. Salas Gismondi, M. Reguero, C. de Muizon, A. Greenwood, B. T. Chait, K. Penkman, M. Collins, and R. D. E. MacPhee. 2019. Palaeoproteomics resolves sloth relationships. Nature Ecology & Evolution 3:1121–1130. doi:10.1038/s41559-019-0909-z
Pujos, F., T. J. Gaudin, G. De Iuliis, and C. Cartelle. 2012. Recent advances on variability, morpho-functional adaptations, dental terminology, and evolution of sloths. Journal of Mammalian Evolution 19(3):159–169. doi:10.1007/s10914-012-9189-y
Pujos, F., G. De Iuliis, and C. Cartelle. 2016. A paleogeographic overview of tropical fossil sloths: towards an understanding of the origin of extant suspensory sloths? Journal of Mammalian Evolution 24(1):19–38. doi:10.1007/s10914-016-9330-4
Resar, N. A., J. L. Green, and R. K. McAfee. 2013. Reconstructing paleodiet in ground sloths (Mammalia, Xenarthra) using dental microwear analysis. Kirtlandia 58:61–72.
Román-Carrión, J. L., L. Brambilla. 2019. Comparative skull osteology of Oreomylodon wegneri (Xenarthra, Mylodontinae): defining the taxonomic status of the Ecuadorian endemic mylodontid. Journal of Vertebrate Paleontology 39(4):e1674860. doi:10.1080/02724634.2019.1674860
Sánchez Chillón, B., J. L. Prado, and M. T. Alberdi. 2003. Paleodiet, ecology, and extinction of Pleistocene gomphotheres (Proboscidea) from the Pampean Region (Argentina). Coloquios de Paleontología 1:617–625
Schaub, S. 1935. Säugetierfunde aus Venzuela und Trinidad. Abhandlungen der Schweizerischen Palaeontologischen Gessellschaft 55:1–21.
Segall, W. 1970. Morphological parallelisms of the bulla and auditory ossicles in some insectivores and marsupials. Fieldiana, Zoology 51:169–205.
Steadman, D. W., P. S. Martin, R. D. E. MacPhee, A. J. T. Jull, H. G. McDonald, C. A. Woods, M. Iturralde–Vinent, and G. W. L. Hodgins. 2005. Asynchronous extinction of late Quaternary sloths on continents and islands. Proceedings of the National Academy of Sciences 102:11763–11768. doi:10.1073/pnas.0502777102
Varela, L., P. S. Tambusso, H. G. McDonald, and R. A. Fariña. 2018. Phylogeny, macroevolutionary trends and historical biogeography of sloths: insights from a Bayesian morphological clock analysis. Systematic Biology 68(2):204–218. doi:10.1093/sysbio/syy058
Vizcaíno, S. F., G. H. Cassini, J. C. Fernicola, and M.S. Bargo. 2011. Evaluating habitats and feeding habits through ecomorphological features in glyptodonts (Mammalia, Xenarthra). Ameghiniana 48(3):305–319. doi:10.5710/AMGH.v48i3(364)
Webb, S. D. 1991. Ecogeography and the Great American Interchange. Paleobiology 17(3):266–280.
Wible, J. R. 2010. Petrosal anatomy of the nine-banded armadillo, Dasypus novemcinctus Linnaeus, 1758 (Placentalia: Xenarthra: Dasypodidae). Annals of Carnegie Museum 79:1–28. doi:10.2992/007.079.0101.
Wing, E. S. 1962. Succession of mammalian faunas on Trinidad, West Indies. Unpublished M.S. thesis. University of Florida, Gainesville. 76 p.
Woodburne, M. O., F.J. Goin, M. Bond, A. A. Carlini, J. N. Gelfo, G. M. López, A. Iglesias, and A. N. Zimicz. 2014. Paleogene land mammal faunas of South America; a response to global climatic changes and indigenous floral diversity. Journal of Mammalian Evolution 21(1):1–73. doi:10.1007/s10914-012-9222-1